

Your doctor has prescribed Zematop®
to help relieve your eczema
Zematop® is used for the treatment of moderate to severe eczema (atopic dermatitis) in people over the age of 16 years, who need a longer term non-steroid based treatment or have not had any success using other treatments such as corticosteroid creams and ointments.
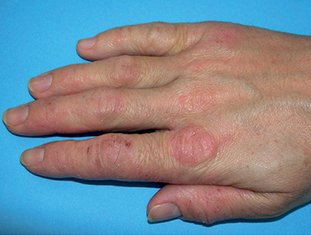
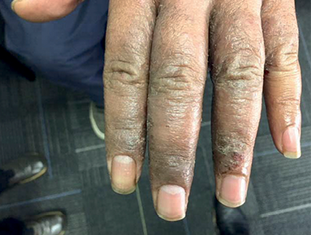
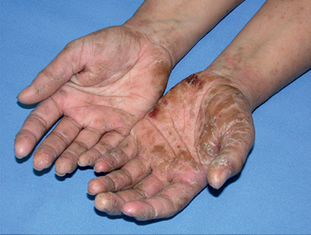
Pictures published with permission of Dermnet
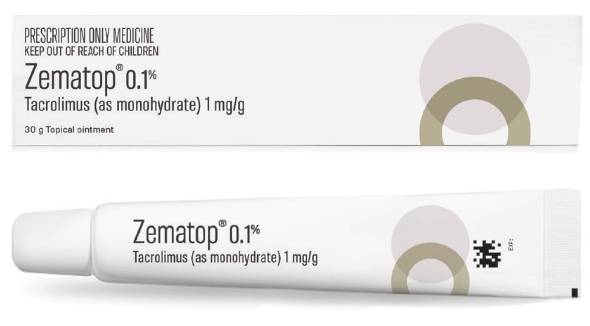
Zematop® (tacrolimus in a 10g, 30g or 60g tube) is now available on prescription for severe atopic dermatitis in adults and adolescents over the age of 16 years who are not adequately responsive to or are intolerant of conventional therapies such as topical corticosteroids.1
Zematop® is a prescription medicine for the treatment of moderate to severe atopic dermatitis in adults and adolescents over the age of 16 who are not adequately responsive or who are intolerant of conventional therapies such as topical steroids. Ingredient is tacrolimus 0.1% Dose. Maintenance. Apply once a day twice weekly to areas commonly affected by atopic dermatitis to prevent progression to flares. Between applications there should be 2-3 days without Zematop® treatment. After 12 months treatment there should be a review of the patient’s condition. There is an absence of safety data beyond 12 months. Flares. Apply twice a day and treatment should be continued until clearance of lesion. If symptoms recur, twice daily treatment should be restarted. Contraindications. Hypersensitivity to macrolides in general. Occlusive dressings are not recommended. Warnings and Precautions. Minimise exposure to sunlight and use of UV light from a solarium in combination with psoralens (PUVA). Use in patients with skin barrier defects such as Netherton’s syndrome or cutaneous graft versus host disease. Care if applying Zematop® over an extended period of time. Care with transplant patients re development of lymphadenopathy. This should be investigated and kept under review. In cases of persistent lymphadenopathy the aetiology should be investigated and possible discontinuation of Zematop® should be considered. Before commencing treatment of clinically infected atopic dermatitis the clinical infections at treatment sites should be cleared. Treatment with Zematop® may be associated with an increased risk of herpes viral infections and folliculitis. The balance of risk/benefit should then be evaluated. Avoid contact with eyes and mucous membranes. Patients with hepatic failure as tacrolimus is extensively metabolised in the liver. Pregnancy, breast feeding is not recommended unless clearly necessary. There is no data available on fertility. Interactions. Formal studies have not been conducted. Interactions with CYP 3A4 inhibitors cannot be ruled out. Adverse Effects. Very common: application site burning & pruritis. Common: local skin infection, herpes viral infections, alcohol intolerance, paraethesias and burning sensation, pruritis. Please review the datasheet at www.medsafe.govt.nz before prescribing Zematop®. Funded under special authority conditions only for atopic dermatitis of the face on the Pharmaceutical Schedule. Douglas Pharmaceuticals Ltd, Auckland. TAPS NA 13200 811414
Zematop® is a registered trademark in New Zealand and other countries.